Parmelia sulcata - Hammered Shield Lichen
Family: Parmeliaceae [3]
Parmelia sulcata (Wikimedia)1 |

Parmelia sulcata (Wikimedia)2 |

Parmelia sulcata (Wikimedia)3 |
1. Introduction to Parmelia sulcata
1.1. General Characteristics and Ecological Significance
Parmelia sulcata, first formally described by Thomas Taylor in 1836, stands as one of the most widespread and resilient lichen species found across the globe.[1] This foliose lichen exhibits a remarkable cosmopolitan distribution, thriving in temperate and cold regions of both the Northern and Southern Hemispheres.[1] Its adaptability allows it to colonize diverse substrates, including the bark of trees, decaying wood, and various types of rocks.[1]
Ecologically, P. sulcata represents a complex life form, embodying a symbiotic association between a fungal partner, known as the mycobiont, and a photosynthetic partner, typically green algae, referred to as the photobiont.[1][7] This unique biological partnership enables the lichen to thrive in environments where neither organism could survive independently.[7] A particularly significant ecological role of P. sulcata is its function as a bioindicator.[8] Its distinctive capacity to accumulate pollutants such as heavy metals and radionuclides directly reflects ambient air quality and contamination levels, making it an invaluable tool in pollution studies across continents.[1][9] Beyond its environmental monitoring capabilities, P. sulcata also holds historical importance, having been utilized in traditional medicine and as a source of natural dyes for centuries.[1]
1.2. Taxonomic History and the Parmelia sulcata Species Complex
The taxonomic classification of Parmelia sulcata has a complex history.[10] Taylor's initial description in 1836 characterized its thallus as orbicular and stellate, with a glaucous white to grey color when dry and a darker underside, noting its distinctive reticulated pattern and the presence of oblong or linear eruptions of soredia.[1][11] These morphological features were historically used for identification.[11]
However, recent advancements in molecular phylogenetics have unveiled a more intricate reality.[12] Studies have demonstrated significant genetic variability within what was traditionally considered a single species, revealing that P. sulcata is, in fact, a complex of cryptic species.[1][13] This complex includes genetically distinct lineages such as Parmelia encryptata and P. barrenoae, highlighting the critical necessity of molecular data for accurate species identification.[1] The term P. sulcata sensu stricto (s. str.), meaning "in the strict sense," is now applied to the lineage encompassing the majority of specimens found across Europe, Asia, and North America, including those from its lectotype locality in Ireland.[13] Parmelia encryptata, while morphologically similar, is genetically distinct.[3] This discovery underscores a broader pattern in mycology where species diversity has often been underestimated, particularly in widely distributed taxa.[3]
The recognition of P. sulcata as a species complex carries profound implications, particularly for its established role as a bioindicator.[14] If distinct cryptic species within the P. sulcata aggregate exhibit different physiological responses to pollutants or varying environmental changes, then past bioindication studies that did not differentiate these species might have aggregated data from biologically distinct entities.[15] This could lead to conclusions regarding pollution levels or ecological impacts that are less precise or even inaccurate.[16] For instance, one cryptic species might be more sensitive to heavy metals, while another might be more tolerant to sulfur dioxide.[17] Without molecular identification, a study might misinterpret the overall health of an ecosystem or the specific impact of a pollutant.[18] This situation necessitates a re-evaluation of historical bioindication data and strongly suggests that future environmental monitoring efforts should incorporate molecular identification techniques to ensure scientific rigor and enhance the accuracy of interpreting environmental contamination.[19] The widespread distribution of P. sulcata, rather than indicating a single highly adaptable species, appears to be a testament to the success of a closely related group of species, each potentially possessing subtly different ecological niches or tolerances.[20]
2. Biological Profile
2.1. Morphology and Anatomy
Parmelia sulcata is characterized as a foliose lichen, meaning it has a leaf-like growth form that is generally loosely attached to its substrate.[21] Its thallus typically forms a circular shape, ranging in diameter from 6 cm up to 10 cm.[1][22] The upper surface of the thallus varies in color from a glaucous white to a dull grayish-green or grey, while the lower surface is distinctly black.[1] The thallus is broadly lobed, with individual lobes measuring between 2–5 mm in width, and these lobes often overlap.[1]
A key morphological feature distinguishing P. sulcata is the presence of both marginal and laminal soralia.[22] These are oblong or linear eruptions on the thallus surface that release soredia—fine, greyish-brown powdery propagules essential for asexual reproduction.[1][23] Additionally, elongated pseudocyphellae, which are small pores in the cortex, form a distinctive reticulate pattern across both the laminal (surface) and marginal regions of the lobes.[1] On the underside of the thallus, root-like structures called rhizines are present, ranging from simple (unbranched) to squarrose (brush-like) in form.[1] When present, sexual reproductive structures called apothecia are typically central and substipitate, measuring up to 8 mm in diameter.[23] They are lecanorine in form, featuring a red-brown disc, and often possess a sorediate margin.[1][24] Standard chemical spot tests performed on the upper surface of P. sulcata yield a K+ (yellow) reaction, while remaining KC−, C−, and P−.[24] The medulla and soredia, however, react K+ (red-orange) and Pd+ (orange).[25] These reactions are indicative of the presence of specific secondary metabolites, such as atranorin and chloroatranorin.[2][26]
2.2. Symbiotic Relationships: The Mycobiont and Photobiont Partnership
Lichens are classic examples of dual organisms, representing a highly integrated symbiotic association between a fungal partner (mycobiont) and a photosynthetic partner, which can be either an alga or a cyanobacterium (photobiont).[4] In the case of Parmelia sulcata, the primary photobiont is consistently identified as a green microalga belonging to the genus Trebouxia, specifically species within Trebouxia clade I.[1][27]
Research indicates that P. sulcata maintains a remarkably specific relationship with a limited number of Trebouxia lineages, including Trebouxia sp. I02, T. flava, and Trebouxia aff. flava.[1][28] This high degree of specificity stands in contrast to some other Parmelia species, which are known to associate with a wider array of Trebouxia species.[1] The biodiversity patterns observed in the photobionts of Parmelia are shaped by a complex interplay of ecological, climatic, and evolutionary factors.[1]
The highly specific photobiont relationship maintained by P. sulcata presents an intriguing paradox when considering its widespread, cosmopolitan distribution across diverse temperate and cold regions globally.[1] Intuitively, a species with such a broad geographical range might be expected to exhibit a more flexible or generalized symbiotic relationship, allowing it to adapt to a wider array of environmental conditions by associating with various photobionts.[29] However, P. sulcata's narrow specificity directly contradicts this common ecological expectation for widespread species.[30] This observation suggests that the global success of P. sulcata (or its aggregate) is not attributable to a broad choice of photobionts, but rather to other factors.[31] These could include the inherent adaptability of Trebouxia clade I itself, which might possess a remarkable tolerance to diverse microclimates;[32] the mycobiont's robust ability to thrive and form a functional symbiosis despite a restricted range of photobiont options;[33] or that the extensive distribution is primarily driven by the efficient dispersal of this highly specific symbiotic unit, rather than the formation of new symbiotic partnerships in novel environments.[34] This narrow specificity could also render P. sulcata more vulnerable to environmental changes that specifically impact its preferred Trebouxia lineages, potentially affecting its long-term adaptability despite its current resilience.[35] Further research is warranted to fully understand the physiological advantages conferred by Trebouxia clade I that enable P. sulcata's global success, and to explore how this specificity might influence its response to rapid environmental shifts.[36]
2.3. Reproductive Strategies
Parmelia sulcata primarily reproduces asexually through the dispersal of soredia.[1][37] Soredia are specialized vegetative propagules that are crucial for the lichen's dispersal, as they contain both the fungal hyphae and algal cells, ensuring that the entire symbiotic unit is spread to new locations.[1] This method of reproduction is highly efficient for colonizing new substrates and for rapid proliferation in suitable environments.[37]
While asexual reproduction is dominant, apothecia, the sexual reproductive structures, are not exceptionally rare, particularly in rural areas.[1][38] This indicates that P. sulcata also engages in sexual reproduction, producing ascospores that typically measure between 8–14 μm.[1] The vegetative reproduction through soredia is thought to play a role in the selection of compatible photobionts, as the pre-packaged symbiotic unit ensures the continued association with specific algal partners.[1] Notably, studies have revealed high genetic variability within P. sulcata, even within lineages that primarily reproduce vegetatively.[38] This suggests that lichens, even without frequent sexual reproduction, may still engage in mechanisms like the exchange of photobionts, which can contribute to their observed genetic diversity.[3][39]
3. Global Distribution and Ecological Role
3.1. Geographic Range and Habitat Preferences
Parmelia sulcata is a truly cosmopolitan species, exhibiting a global distribution across temperate and cold regions in both the Northern and Southern Hemispheres.[1][40] In North America, its presence is described as "extremely widespread, even weedy," extending southward into Mexico's Baja California.[1] Similarly, in Europe, it is recognized as one of the most common parmelioid lichens, having been recorded in 43 countries.[1]
Despite its prevalence in the Northern Hemisphere, P. sulcata is comparatively rare in South America.[40] However, recent molecular evidence from a 2023 study confirmed its presence in Chile, with DNA sequences matching the most common haplotype found across Europe, Asia, and North America, indicating a broad distribution of this particular genetic lineage.[1][41] In Africa, P. sulcata has been documented in high-elevation low alpine zones of Ethiopia and Kenya, at altitudes ranging from 3,500 to 4,200 meters (11,500 to 13,800 feet), but it remains uncommon elsewhere on the continent.[1]
Regarding its habitat preferences, P. sulcata demonstrates versatility, growing on a variety of substrates including tree bark, wood, and rocks.[1] While it shows a general preference for bark or wood, it can also be found on siliceous or mossy rocks.[1][42]
3.2. Environmental Factors Influencing Distribution
The widespread distribution of Parmelia sulcata is influenced by a combination of environmental factors:
- Climate and Temperature: The species thrives in temperate to cold regions, indicating its strong adaptability to cooler environments.[2][43] This climatic preference largely dictates its global range.[43]
- Pollution Resilience: P. sulcata is notably resilient to pollution.[44] Its observed reappearance in temperate urban areas following reductions in sulfur dioxide levels highlights its capacity to tolerate and recover from certain anthropogenic stressors.[1][45]
- Substrate Versatility: Its ability to colonize diverse surfaces, including bark, wood, and rocks, significantly contributes to its broad distribution across varied landscapes.[2]
- Light Conditions (Insolation): Studies indicate that P. sulcata is most abundant in the middle to upper parts of tree trunks, particularly in areas exposed to higher light levels.[46] This suggests that insolation, or exposure to sunlight, is a significant factor influencing its growth and distribution.[46] For example, a Russian study found it frequently at heights of 10–20 meters on birch trees and around 15 meters within the crowns of spruce trees, reflecting its preference for higher insolation in these zones.[1][47]
- Microclimatic Conditions: The vertical zonation of P. sulcata along tree trunks is further influenced by various microclimatic conditions, including humidity, light availability, and the chemical and physical properties of the bark.[48] The species composition of lichens, including P. sulcata, changes from the base to the crown of trees, emphasizing the importance of these localized conditions.[1]
- Altitude and Glacier Proximity: While P. sulcata is a foliose lichen, its presence at high altitudes in Africa suggests that factors like absolute altitude can be influential, similar to their importance for crustose lichens.[48][49] For fruticose lichens, slope exposure and steepness are critical.[1]
The interplay between P. sulcata's resilience to pollution and its specific climatic preferences creates a complex dynamic, particularly in the context of ongoing global climate change.[49] Its notable pollution tolerance might enable it to persist in urban environments even as climatic zones shift, potentially positioning it as a "survivor" species in landscapes increasingly altered by human activity.[50] However, its inherent preference for temperate to cold regions suggests that broader warming trends could lead to contractions at its southern range limits or in lower altitude zones, despite its ability to withstand local pollution.[51] This situation illustrates a scenario where the species' strength in one adaptive area (pollution tolerance) may not fully compensate for its vulnerability in another (temperature sensitivity).[52] The result is not a simple expansion or contraction, but rather a complex redistribution of its populations.[53] This makes P. sulcata an exceptionally valuable biological model for investigating how multiple environmental stressors—such as pollution, temperature fluctuations, and light availability—interact to shape species distributions under the pressures of global change.[54]
3.3. Parmelia sulcata as a Bioindicator of Environmental Pollution
Lichens, including Parmelia sulcata, are widely recognized as invaluable biosensors and bioindicators for environmental pollution.[55] This capacity stems from their unique physiological characteristics: they lack true roots and a protective cuticle, absorbing nutrients and pollutants directly from the atmosphere and precipitation.[4][56] This makes them highly sensitive to environmental fluctuations and allows them to accumulate various substances from their surroundings.[1]
P. sulcata is particularly adept at accumulating pollutants such as heavy metals and radionuclides, making it an excellent proxy for assessing ambient air quality and contamination levels.[1] Its sensitivity extends to a range of atmospheric pollutants, including sulfur dioxide, oxides of nitrogen, fluorides, photochemical toxins, and various heavy metals.[4] The observation that P. sulcata recolonizes urban areas after reductions in sulfur dioxide levels further highlights its sensitivity and utility as a recovery indicator.[1][57]
Various methods are employed to leverage P. sulcata's bioindicator capabilities.[57] These include mapping the distribution and abundance of the species in a particular site, individual sampling and chemical analysis of accumulated pollutants within its tissues, and transplanting lichen samples from uncontaminated areas to polluted sites for subsequent analysis of morphological changes and bioaccumulation of toxins.[4][58]
Numerous studies demonstrate its effectiveness:
- A national monitoring survey in The Netherlands successfully utilized P. sulcata as a bioindicator for trace-element air pollution.[4]
- In Portugal, studies involving transplanted P. sulcata samples near fuel and coal-powered plants showed high accumulation of heavy metals, effectively identifying emission sources.[4]
- Research along a highway in Central Lithuania used P. sulcata to monitor traffic pollution, observing a direct correlation between nitrogen dioxide levels and nitrogen accumulation in the lichen's tissues.[4][59]
- In Serbia, P. sulcata was employed as a bioindicator for heavy metal pollution, demonstrating a tendency to accumulate manganese (Mn), nickel (Ni), titanium (Ti), and iron (Fe).[4]
- Morphological changes, such as necrosis, size reduction, and color alterations, observed in P. sulcata specimens in urban environments in Pskov, Russia, provided visual evidence of pollution impact.[4]
- Studies in the USA also reported a significant decline in the algal layer ratio and thallus thickness in P. sulcata specimens collected from polluted localities, directly attributing these changes to air pollution.[4]
4. Chemical Composition and Secondary Metabolites
4.1. Identification of Key Lichen Products (e.g., Atranorin, Salazinic Acid)
Parmelia sulcata is known to produce a diverse array of distinctive chemical constituents, particularly secondary metabolites that are unique to lichens.[4][60] These compounds are not only crucial for the lichen's survival in harsh environments but also form the basis of its observed pharmacological activities.[60]
Key lichen products consistently identified in P. sulcata include:[61]
- Salazinic acid: This is a major metabolite, classified as a β-orcinol-type depsidone.[3] It has garnered significant attention due to its demonstrated high cytotoxic activity, particularly in human melanoma and colon cancer cell lines.[7]
- Atranorin: Another prominent component, atranorin is frequently found alongside salazinic acid.[3]
- Chloroatranorin: Often identified in conjunction with atranorin.[2]
- Protocetraric acid: Mentioned in more recent studies as a constituent.[7]
- Consalazinic acid: Also reported to be present.[3]
- Lobaric acid: This compound is sometimes found in P. sulcata.[7]
The presence of these secondary metabolites is further corroborated by standard spot tests.[61] The upper surface of P. sulcata reacts K+ (yellow), while the medulla and soredia yield K+ (red-orange) and Pd+ (orange) reactions, all indicative of these compounds.[2][62] These lichen products are largely responsible for the broad spectrum of pharmacological activities observed in P. sulcata extracts.[4]
4.2. Analysis of Volatile Organic Compounds and Other Constituents
Beyond the well-known lichen products, comprehensive analysis using techniques such as gas chromatography–time of flight mass spectrometry (GCXGC–TOF/MS) has provided a more detailed chemical fingerprint of P. sulcata extracts (PSE).[7][63] This advanced method is particularly valuable for its ability to provide swift qualitative and quantitative analysis of volatile compounds from relatively small sample amounts.[7]
The GCXGC–TOF/MS analysis identified 26 distinct compounds in the P. sulcata extract.[63][64] The major components, quantified as a percentage of the total ion chromatogram peak area, are presented in Table 1 below.[64][65]
Table 1: Major Chemical Constituents Identified in Parmelia sulcata Extracts[66]
| Compound Name | Chemical Class (if applicable) | Relative Percentage (%) |
|---|---|---|
| Eicosane | Alkane | 24.43 |
| Methyl 2,4-dihydroxy-3,6-dimethylbenzoate | Ester | 20.65 |
| 2-Methylnonadecane | Alkane | 16.18 |
| (E,E)-3,7,11-trimethyl-2,6-dodecadien-1-ol | Alcohol | 5.60 |
| 2-Methyloctadecane | Alkane | 5.28 |
| Heneicosane | Alkane | 4.66 |
| 9-Octadecenal | Aldehyde | 4.65 |
| 3,7,11,15-Tetramethyl-2-hexadecen-1-ol | Alcohol | 2.54 |
| n-Hexadecanoic acid | Fatty Acid | 2.43 |
| Acetic acid | Carboxylic Acid | 0.94 |
| 1-Heneicosanol | Alcohol | 1.00 |
| 1-Docosanol, acetate | Ester | 1.59 |
| Farnesane | Sesquiterpene | 1.33 |
| (Other 13 identified compounds) | Various | < 2.00 (each) |
| Unknown | 4.63 | |
| Total Major Lichen Products (Identified through various analyses)[68] | ||
| Salazinic acid | Depsidone | (Major)[3][7] |
| Atranorin | Depside | (Major)[3] |
| Chloroatranorin | Depside | (Major)[2] |
| Protocetraric acid | Depsidone | (Present)[7] |
| Consalazinic acid | Depsidone | (Present)[3] |
| Lobaric acid | Depsidone | (Sometimes Present)[7] |
Note: Percentages for specific volatile compounds are from GCXGC-TOF/MS analysis of the extract.[67] Salazinic acid, atranorin, and related compounds are major lichen products identified through other analyses and contribute significantly to the extract's properties, though their exact percentages in the specific volatile fraction may vary or not be explicitly stated in all analyses.[7][68][69] All other identified components were present at concentrations less than 2%.[7] Beyond these specific compounds, lichens in general are known to synthesize a wide array of other secondary metabolites, including various depsides, depsidones, phenols, polysaccharides, lipids, ethers, and dibenzofurans.[6] Many of these diverse metabolites are collectively responsible for the broad pharmacological activities observed in lichen extracts.[10]
The chemical analysis of P. sulcata reveals a compelling aspect concerning the dual nature of its bioactive compounds: their potency versus their safety.[69] While major components like salazinic acid are highlighted for their significant cytotoxic activity against cancer cell lines[4][70], it is also noted that P. sulcata extracts can induce caspase-independent apoptotic cell death at lower concentrations, but may exhibit genotoxic activity at relatively higher doses.[7] This observation points to a critical dose-dependent effect, suggesting a potentially narrow therapeutic window for some of these compounds.[70] For therapeutic development, this necessitates extremely careful consideration. Rigorous dose-response studies and comprehensive toxicology assessments are paramount to precisely define the effective and safe concentration ranges.[71] This approach is essential to maximize the therapeutic benefits while simultaneously minimizing any harmful side effects.[72] This dual nature of potency and potential toxicity is a common challenge encountered in the discovery and development of drugs from natural products, emphasizing the need for thorough scientific scrutiny beyond initial promising in vitro findings.[73]
5. Applications of Parmelia sulcata
5.1. Traditional Uses in Medicine, Dyes, and Other Cultural Practices
For centuries, Parmelia sulcata has been integrated into various traditional practices across different cultures, demonstrating its historical significance beyond its ecological role.[1][74]
In traditional medicine, P. sulcata was notably employed for:
- Cranial Disorders: It was traditionally used to cure cranial disorders.[74] This use was notably influenced by the "Doctrine of Signatures" in the 15th century, which posited that plants revealed their medicinal properties through their morphology;[4][75] P. sulcata's convoluted appearance was thought to resemble a human brain, leading to its application for mental and cranial ailments.[4][76]
- Teething Babies: The Metis people of Alberta, Canada, traditionally rubbed P. sulcata on the gums of teething babies to alleviate discomfort.[4]
- Birth Control: The Saanich community in British Columbia, Canada, possibly used this lichen for birth control, with its medicinal properties believed to depend on the type of tree it grew on.[4]
- Skin Diseases: The historical correlation of the Greek word 'leprous' with lichens suggests their use in alleviating skin diseases, possibly due to their peeling skin-like appearance.[4]
Beyond medicinal applications, P. sulcata also served practical purposes:[76][77]
- Dyeing: Lichen-derived natural colorants were extensively utilized in European countries for dyeing purposes during the Middle Ages.[4] P. sulcata specifically has been used to dye wool, yielding a range of hues from clear yellow to yellowish and dark, even rusty brown.[4]
- Mummification: In Ancient Egypt, fragrant lichens were significant ingredients in the process of mummification.[4]
While P. sulcata is not explicitly listed as a common human food source in all available information, lichens in general have a history of consumption due to their valuable nutrients.[4] Examples of edible lichens include Umbilicaria esculenta and Cetraria islandica, which were important human foods in various cultures.[4][78] Some cultures consider lichens a staple food or even a delicacy, though they have often served as famine foods during times of dire need.[14] Certain lichens are also known to be a source of vitamin D and are rich in proteins, vitamins, enzymes, and polysaccharides.[5]
5.2. Modern Medicinal and Pharmacological Potential
Modern scientific inquiry has begun to validate and expand upon the traditional uses of Parmelia sulcata, revealing a range of promising pharmacological activities attributable to its unique chemical constituents.[79]
5.2.1. Anticancer Activity and Proposed Mechanisms of Action[80]
P. sulcata contains salazinic acid, atranorin, and various volatile oils that contribute to its observed anticancer potential.[4]
- Induction of Apoptosis and DNA Damage: Methanolic extracts of P. sulcata have been shown to induce caspase-independent apoptosis and exhibit genotoxic activity at higher doses in breast cancer cell lines, specifically MCF-7 and MDA-MB-231.[4] For MCF-7 cells, the IC50 (half-maximal inhibitory concentration) was determined to be 39.1 μg/ml, and for MDA-MB-231 cells, it was 16.5 μg/ml.[7] These extracts also induced cell death via DNA damage in other cancer cell lines, including human lung (A549), prostate (PC3), liver (Hep3B), and rat glioma (C6).[4][81]
- Selective Apoptosis: Silver nanoparticles (Ps-AgNPs) synthesized from the phenolic extract of P. sulcata have demonstrated the ability to selectively induce apoptosis in MCF-7 cancer cells, indicating a targeted approach to cancer treatment.[4]
- Glioblastoma Activity: Acetone extracts of P. sulcata, rich in salazinic acid (approximately 23.05%), have shown activity against glioblastoma (GBM) cell lines (A-172, T98G), decreasing cell viability at concentrations as low as 50 µg/mL.[4]
- General Anti-proliferative Effects: The methanolic extracts have exhibited general anti-proliferative effects across various human cancer cell lines.[4]
- Mechanism: A notable mechanism identified is the induction of caspase-independent apoptosis, suggesting a distinct pathway for cell death.[7][82]
5.2.2. Antimicrobial Properties (Antibacterial and Antifungal)
Extracts of P. sulcata (including petroleum ether, acetone, chloroform, diethyl ether, and methanol) and its constituent salazinic acid exhibit significant antimicrobial activity against a range of food-borne bacteria and fungi.[4]
- Broad-Spectrum Activity: These extracts are effective against various bacteria, including Aeromonas hydrophila, Bacillus cereus, Bacillus subtilis, Staphylococcus aureus, and Escherichia coli, as well as fungi such as Candida albicans and Penicillium notatum.[4]
- Active Components: Salazinic acid and atranorin have been identified as major active components responsible for these antimicrobial effects.[4] Furthermore, flavonoids and phenolics present in ethyl acetate fractions of P. sulcata also contribute to its antibacterial and antifungal activities.[4][83]
- Effectiveness: Acetone and methanol extracts have demonstrated prominent inhibitory activity against most tested microorganisms.[83] The acetone extract, for instance, showed the largest zones of inhibition against B. subtilis (25 mm) and E. coli (24 mm).[4][84]
- Specificity: While generally effective against Gram-positive bacteria, some extracts were found to be ineffective against Gram-negative bacteria.[12] However, salazinic acid specifically showed activity against Pseudomonas aeruginosa and Salmonella typhimurium.[12]
5.2.3. Antioxidant Activity[85]
P. sulcata extracts demonstrate significant antioxidant potential.[4]
- DPPH Radical Scavenging: Methanolic extracts have shown antioxidant potential through the DPPH radical scavenging assay, correlating with their high flavonoid and phenolic content.[4] The acetone extract, for example, contained 38.2 ± 1.27 μg of pyrocatechol equivalent phenolics and 25.8 ± 1.11 μg of rutin equivalent flavonoids.[11] These compounds are recognized for their crucial role in antioxidant activity.[11]
- Superoxide Anion Scavenging: Aqueous extracts of P. sulcata have also demonstrated superoxide anion scavenging activity, with a reported activity of 12.74%.[4]
5.2.4. Other Pharmacological Activities (e.g., Mosquitocidal)[86]
Beyond the extensively studied anticancer, antimicrobial, and antioxidant properties, P. sulcata exhibits other promising pharmacological activities:
- Mosquitocidal: Gold nanoparticles (AuNPs) synthesized using the aqueous extract of P. sulcata have shown promising mosquitocidal activity.[86] These AuNPs were found to be toxic to pupae, egg-hatching larvae, and adults of Anopheles stephensi and Aedes aegypti, indicating potential for vector control.[4][87]
- Anti-inflammatory/Antipyretic/Analgesic: While some lichens are generally known for anti-inflammatory, antipyretic, and analgesic properties[7], specific detailed studies or results for P. sulcata in these areas are not provided in the available information.[11]
The progression from the traditional uses of P. sulcata to its modern scientific validation, and further to applications in nanomedicine, highlights a remarkable spectrum of therapeutic potential.[87] Ancient cultures utilized P. sulcata empirically for conditions such as cranial disorders and teething pain.[4][88] Modern research has systematically validated its extracts for anticancer, antimicrobial, and antioxidant activities, identifying specific bioactive compounds.[4] The subsequent development of silver and gold nanoparticles synthesized using P. sulcata extracts for targeted cancer therapy and mosquitocidal effects[4] represents a significant leap into advanced biomedical applications.[88][89] This trajectory demonstrates P. sulcata's enduring value, evolving from traditional folk medicine to sophisticated modern pharmacology and even nanotechnology.[89] It suggests that ethnobotanical knowledge can serve as a powerful guide for identifying species with substantial bioactive potential.[90] The integration of nanomedicine indicates a future where P. sulcata's active compounds could be delivered with enhanced precision and efficacy, potentially mitigating issues like poor bioavailability or non-specific toxicity.[91] This synergy between traditional wisdom and cutting-edge scientific methods positions P. sulcata as a prime candidate for continued drug discovery and development, particularly in fields such as oncology and vector control.[92]
5.3. Food and Industrial Applications[93]
Beyond its medicinal and environmental roles, Parmelia sulcata and lichens in general have found applications in food and industry.[93]
- Food: While P. sulcata is not explicitly cited as a common human food source in the provided information, lichens broadly have a history of consumption due to their valuable nutrient content.[4][94] Examples of edible lichens include Umbilicaria esculenta and Cetraria islandica, which were historically significant food sources in various cultures, sometimes serving as staples or delicacies, though often as famine foods.[4] Lichens are recognized for being rich in proteins, vitamins, enzymes, and polysaccharides, and some species are even a source of vitamin D.[5]
- Industrial (Dyes): P. sulcata has been historically utilized as a source of natural dyes.[1] Lichen-derived natural colorants were extensively employed in European countries for dyeing purposes during the Middle Ages.[4][95] Specifically, P. sulcata is known for its ability to dye wool, producing a range of hues from clear yellow to yellowish and dark, even rusty brown.[4]
5.4. Detailed Environmental Applications (Biomonitoring)[96]
P. sulcata's role as a bioindicator is one of its most significant environmental applications.[96] Its unique physiology, lacking a protective cuticle and true roots, means it absorbs nutrients and pollutants directly from the atmosphere, making it an excellent sentinel for environmental health.[4][97]
- Pollutant Accumulation: It possesses a remarkable capacity to accumulate heavy metals and radionuclides, thereby providing a direct reflection of ambient air quality and contamination levels.[1]
- Sensitivity: The species is highly sensitive to a range of atmospheric pollutants, including sulfur dioxide (SO2), oxides of nitrogen, fluorides, photochemical toxins, and various heavy metals.[4] Its observed recolonization of urban areas after reductions in SO2 levels further underscores its sensitivity and utility as an indicator of environmental recovery.[1][98]
- Monitoring Methods: Environmental monitoring utilizing P. sulcata can involve several approaches: mapping the species' presence and abundance in a given area, collecting individual samples for direct chemical analysis of accumulated pollutants, or transplanting samples from pristine environments to potentially contaminated sites to monitor changes in morphology and bioaccumulation over time.[4]
- Case Studies:
- The Netherlands: A national monitoring survey successfully used P. sulcata as a bioindicator for trace-element air pollution across a vast area.[4]
- Portugal: Studies involving transplanted P. sulcata samples near fuel and coal-powered plants demonstrated high accumulation of heavy metals, effectively identifying specific emission sources.[4]
- Lithuania: Research along a highway in Central Lithuania employed P. sulcata to monitor traffic pollution, revealing a direct correlation between nitrogen dioxide levels and nitrogen accumulation within the lichen's tissues.[4][99]
- Serbia: In Serbia, P. sulcata served as a bioindicator for heavy metal pollution, showing a tendency to accumulate manganese (Mn), nickel (Ni), titanium (Ti), and iron (Fe).[4]
- Russia: In Pskov, Russia, morphological changes such as necrosis, size reduction, and color alterations observed in P. sulcata specimens in urban environments provided clear visual evidence of pollution impact.[4]
- USA: Studies in the USA reported a significant decline in the algal layer ratio and thallus thickness in P. sulcata specimens collected from polluted localities, directly attributing these changes to air pollution.[4][100]
6. Cultivation Methods and Challenges
6.1. Current Techniques for Asexual Propagation and Pure Culture Isolation[101]
Developing effective culturing systems for lichens like Parmelia sulcata is crucial for producing identifiable developmental stages, which can then be used for various scientific investigations.[17]
- Asexual Propagation (Soredia): P. sulcata soredia can be successfully dispersed onto plastic cover slips and cultivated outdoors under favorable environmental conditions.[17] Under these conditions, aposymbiotic hyphal extensions, which are crucial for attachment to the substrate, typically appear within two weeks, or usually within five weeks.[17] Further development, including pigmentation, the formation of rhizines, and epicortical development, has been observed within six months.[17] A particularly effective technique for soredia dispersal involves tapping thalli directly onto dry cover slips, which has been found to distribute propagules more evenly than methods using liquid media.[17][102] The transparency of these cover slips offers a significant advantage, allowing for rapid microscopic monitoring of large numbers of propagules and enabling observation of development on the lower surface.[17]
- Pure Culture Isolation (Mycobiont and Photobiont): The isolation of both the fungal (mycobiont) and algal (photobiont) partners of lichens into pure cultures is a feasible, albeit often challenging, endeavor.[18] For P. sulcata, methods involving soredia and direct thallus fragments have proven suitable for obtaining pure fungal cultures.[18] The isolated fungal partner of P. sulcata has been successfully sequenced and assigned a GenBank-Number (OK491792), while its isolated algal partner has been identified as Coccomyxa sp. (GenBank-Number OK491799).[18][103][104] It has been observed that lichen symbionts, when cultivated as cell aggregates under laboratory conditions, tend to grow faster than they do in their natural environments.[18]
6.2. Environmental Conditions Conducive to Cultivation[105]
Successful cultivation of P. sulcata is highly dependent on specific environmental conditions that mimic its natural habitat:
- Humidity and Temperature: Optimal combinations of humidity and temperature are critical for routine culture.[17] Studies suggest that high humidity, specifically above 90%, coupled with cooler temperatures, ideally below 12°C, are particularly conducive to soredial development.[17]
- Light Conditions: In experimental setups, cover slips are often held at a 45-degree angle, which simulates the natural growth angle observed on tree branches.[105][106] Exposure to a north-western direction is also considered, reflecting typical light exposure in its natural environment.[17]
- Substrate: Plastic cover slips have proven to be a suitable substrate for cultivation.[106][107] They are simple to use, inexpensive, and portable, offering a practical solution for laboratory and outdoor experimental setups.[17]
6.3. Challenges and Limitations in Large-Scale Cultivation[108]
Despite the success in small-scale cultivation and isolation of P. sulcata symbionts, significant challenges persist when attempting to scale up these processes for commercial or extensive research purposes.[108]
- Contamination: One of the primary difficulties in cultivating lichen symbionts is the rapid growth of contaminants, such as bacteria and parasitic fungi, in nutrient-rich laboratory media.[109] These contaminants often outcompete the slower-growing lichen symbionts, making it challenging to maintain pure cultures.[18][110]
- Slow Growth: Even under optimized laboratory conditions, lichen symbionts grow considerably slower compared to many other microorganisms.[18] This inherent slow growth rate poses a major bottleneck for large-scale biomass production.[110]
- Isolation Difficulty: The process of isolating mycobionts from the complex lichen thallus is often a lengthy trial-and-error endeavor.[111] It is associated with a high probability of contamination, prolonged incubation times, and generally low success rates.[18][112]
- Soredia Clumping: During propagation, vigorous loosening of soredia in aqueous solutions or the natural tendency of soredia to clump can delay the outgrowth of attachment hyphae.[112][113] This clumping can inhibit development, suggesting that controlling soredia density might be beneficial in future culturing experiments.[17]
- Environmental Stressors: Unfavorable environmental conditions, such as high temperatures and low humidity (e.g., during July in some regions), can significantly stress the developing lichens and delay their growth.[17]
- Economic Feasibility: A major hurdle for large-scale cultivation, particularly concerning the microalgal photobiont, lies in economic feasibility.[113][114] The high cost of key operations such as harvesting, nutrient supply, and extraction of valuable compounds (e.g., oils for biofuel) can be prohibitive.[114] Harvesting alone can account for up to 50% of the total cost in microalgal biofuel production, rendering current commercial ventures economically unfeasible.[19][115]
- Overall Knowledge Gap: The general understanding of Parmelia lichens, including sustainable harvesting or cultivation methods, remains limited compared to other organisms such as higher plants and non-lichenized fungi.[6]
The ability to successfully cultivate P. sulcata soredia on small plastic cover slips and isolate pure cultures of its symbionts in the laboratory[17] stands in stark contrast to the significant challenges faced in scaling up production to a commercially viable level.[115] This fundamental disparity highlights a critical "scaling up" dilemma. The obstacles are not solely biological, such as slow growth rates and susceptibility to contamination, but also economic, particularly the high operational costs associated with harvesting and providing nutrients, especially for the algal partner.[19] This implies that despite P. sulcata's immense pharmacological potential, its practical application for large-scale production of bioactive compounds is severely constrained until more cost-effective, sustainable, and scalable cultivation methods are developed.[116] Future innovations might need to focus on advanced co-cultivation technologies or bioreactor designs that can more efficiently mimic the complex symbiotic growth conditions found in nature.[117] The acknowledged scarcity of knowledge regarding sustainable harvesting and cultivation methods for Parmelia lichens further underscores this bottleneck, indicating a pressing need for dedicated research in this area.[6][118]
7. Future Research Directions and Knowledge Gaps
The current understanding of Parmelia sulcata reveals a fascinating organism with significant ecological and biotechnological potential, yet several areas warrant deeper investigation to fully unlock its value.[119]
7.1. Unexplored Therapeutic Potential and Mechanisms of Action[120]
While in vitro studies have shown promising anticancer, antimicrobial, and antioxidant activities, the full pharmacological profile of P. sulcata requires more comprehensive scientific assessment.[4] A significant knowledge gap exists in precisely elucidating the mechanisms of action for many of these observed activities, particularly beyond initial apoptosis induction.[120] For instance, while its anticancer effects are noted, the specific molecular pathways targeted by P. sulcata compounds are not yet fully detailed in the available literature.[121] Future research should prioritize in-depth studies on the biological activities of its specialized metabolites, including rigorous in vivo and, eventually, clinical studies, to validate the observed pharmacological activities in a living system.[122] Further research into the detailed molecular mechanisms by which these compounds exert their effects is crucial for drug development.[6][123]
7.2. Advanced Phytochemical Characterization and Bioactivity Validation[124]
The current knowledge on Parmelia lichens is considered "still scarce in comparison to other groups of organisms such as higher plants and other non-lichenized fungi".[6] While major compounds have been identified, a more comprehensive phytochemical characterization of P. sulcata and other Parmelia species is needed.[124] The presence of an "unknown" percentage in chemical analyses[7][125] indicates that there are still compounds to be identified and characterized.[125] Future research should focus on the isolation and structural elucidation of novel compounds, especially those present in smaller percentages, followed by their specific bioactivity testing.[126] This should also include exploring the potential synergistic effects of multiple compounds within the complex extracts, as the combined action of several metabolites might be more potent than individual ones.[127]
7.3. Sustainable Cultivation and Bioproduction Strategies[128]
A critical knowledge gap pertains to the exploration of sustainable harvesting or cultivation methods for P. sulcata if it is to become a valuable source of therapeutic compounds.[6] The significant challenges of large-scale cultivation, including contamination, slow growth rates, and high economic costs, remain largely unresolved.[17][128] Future research must aim to develop optimized in vitro and bioreactor cultivation techniques to overcome these limitations, potentially focusing on cultivating the mycobiont alone or developing co-cultivation strategies that more efficiently mimic the natural symbiotic relationship.[128] Research into cost-effective nutrient supply and innovative harvesting methods for biomass production is also vital, potentially exploring fungal-algal co-cultivation for efficient bio-flocculation, which could significantly reduce harvesting costs.[19][129]
7.4. Deeper Understanding of Ecological Dynamics and Cryptic Diversity[130]
The precise interplay and relative importance of multifactorial influences—such as the symbiotic partner, climatic conditions, and geographical factors—on P. sulcata's biodiversity patterns require further disentanglement.[8] The apparent paradox of its narrow photobiont specificity despite its widespread global distribution warrants dedicated investigation.[1] While cryptic species within the P. sulcata complex have been identified[1], it remains a knowledge gap whether P. sulcata sensu stricto itself harbors further hidden genetic diversity that could be resolved with broader molecular analyses and sampling.[8] The implications of this cryptic diversity for bioindication studies are significant, as different cryptic species may respond distinctly to environmental stressors.[3][131]
Future research should therefore:
- Investigate the evolutionary and ecological reasons behind P. sulcata's specific association with Trebouxia clade I, including the physiological advantages this particular symbiosis confers across its diverse habitats.[8]
- Directly quantify how its sorediate vegetative structures influence the microenvironment within the propagules and, consequently, the selection and maintenance of specific photobiont lineages.[8]
- Conduct more extensive global sampling combined with advanced biogeographical modeling to map the precise distribution patterns of P. sulcata and its associated Trebouxia lineages, correlating these patterns with environmental gradients.[8]
- Refine bioindication protocols to explicitly account for cryptic species, potentially requiring molecular identification as a standard step in environmental monitoring programs.[131][132]
The interconnectedness of taxonomy, ecology, and bioactive potential is a crucial consideration for future studies.[132] The chemical profile, and consequently the pharmacological potential, of P. sulcata may vary significantly between cryptic species or across different ecological niches, influenced by the specific photobiont or the environmental stressors present.[133] For example, a sample of P. sulcata sensu stricto collected from a highly polluted urban area might exhibit a different chemical composition or bioactivity compared to Parmelia encryptata from a pristine rural environment.[134] This understanding necessitates an integrated research approach where precise taxonomic identification and detailed ecological context are intrinsically linked to chemical composition analysis and pharmacological studies.[135] This holistic perspective is essential for robust drug discovery, accurate biomonitoring, and the development of effective conservation strategies for this complex and valuable lichen.[136]
8. Conclusion
Parmelia sulcata stands as a remarkably resilient and globally significant lichen, distinguished by its complex taxonomy, unique morphology, and specialized symbiotic relationships.[137] Its established role as a powerful bioindicator for environmental pollution underscores its ecological importance, though the presence of cryptic species within its complex necessitates refined identification methods for accurate monitoring.[138] The rich array of secondary metabolites, particularly salazinic acid and atranorin, provides a strong chemical foundation for its diverse pharmacological activities, including promising anticancer, antimicrobial, and antioxidant properties, with emerging applications in nanomedicine.[139] This progression from traditional uses to cutting-edge scientific validation highlights its enduring value.[140]
Despite its traditional uses and modern scientific validation, significant challenges persist in scaling up cultivation for commercial or extensive research purposes, primarily due to inherent slow growth rates and economic feasibility concerns related to biomass production.[141] Future research must prioritize comprehensive phytochemical characterization, rigorous in vivo and clinical validation of its therapeutic potential, and the development of sustainable and economically viable cultivation strategies.[142] Furthermore, a deeper understanding of its cryptic diversity and the intricate interplay of ecological factors influencing its distribution and chemical profile will be crucial for unlocking its full potential and ensuring its conservation.[143] Parmelia sulcata exemplifies the untapped biotechnological and ecological value residing within lichen species, warranting continued, integrated scientific exploration.[144]
Works Cited
- Parmelia sulcata - Wikipedia, accessed May 21, 2025, https://en.wikipedia.org/wiki/Parmelia_sulcata
- About Hammered Shield Lichen - Maryland Biodiversity Project, accessed May 21, 2025, https://www.marylandbiodiversity.com/species/4724
- (PDF) Parmelia sulcata (Ascomycota: Parmeliaceae), a sympatric ..., accessed May 21, 2025, https://www.researchgate.net/publication/231793025_Parmelia_sulcata_Ascomycota_Parmeliaceae_a_sympatric_monophyletic_species_complex
- (PDF) Perspectives in Biomonitoring and Pharmacological Aspects ..., accessed May 21, 2025, https://www.researchgate.net/publication/381460762_Perspectives_in_Biomonitoring_and_Pharmacological_Aspects_of_Parmelia_sulcata_Taylor
- A Review on Nutritional Composition, Phytochemistry and Pharmacological Properties of Lichen - Journal of University of Shanghai for Science and Technology, accessed May 21, 2025, https://jusst.org/wp-content/uploads/2024/07/A-Review-on-Nutritional-Composition-Phytochemistry-and.pdf
- Current knowledge on Parmelia genus: Ecological interest ..., accessed May 21, 2025, https://pubmed.ncbi.nlm.nih.gov/31234093/
- Promising anticancer activity of a lichen, Parmelia sulcata Taylor, against breast cancer cell lines and genotoxic effect on human lymphocytes - PubMed Central, accessed May 21, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC4371575/
- Biodiversity Patterns and Ecological Preferences of the ... - Frontiers, accessed May 21, 2025, https://www.frontiersin.org/journals/microbiology/articles/10.3389/fmicb.2021.765310/full
- Influence of Orographic Factors on the Distribution of Lichens in the Franz Josef Land Archipelago - PMC, accessed May 21, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC10819431/
- Chemical compositions of Parmelia sulcata extract | Download Table, accessed May 21, 2025, https://www.researchgate.net/figure/Chemical-compositions-of-Parmelia-sulcata-extract_tbl1_261188239
- Antioxidant properties of some lichen species - PMC, accessed May 21, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC3551121/
- Antimicrobial Activity of Extracts of the Lichen Parmelia sulcata and its Salazinic Acid Constituent - ResearchGate, accessed May 21, 2025, https://www.researchgate.net/publication/5932081_Antimicrobial_Activity_of_Extracts_of_the_Lichen_Parmelia_sulcata_and_its_Salazinic_Acid_Constituent
- (PDF) Lichens Used in Traditional Medicine - ResearchGate, accessed May 21, 2025, https://www.researchgate.net/publication/283808263_Lichens_Used_in_Traditional_Medicine
- Edible lichen - Wikipedia, accessed May 21, 2025, https://en.wikipedia.org/wiki/Edible_lichen
- Parmelia sulcata Taylor and Usnea filipendula Stirt induce apoptosis‐like cell death and DNA damage in cancer cells - PMC, accessed May 21, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC6496759/
- Unassuming Lichens: Nature's Hidden Antimicrobial Warriors - MDPI, accessed May 21, 2025, https://www.mdpi.com/1422-0067/26/7/3136
- (PDF) Growing foliose lichens on cover slips: A method for asexual ..., accessed May 21, 2025, https://www.researchgate.net/publication/273101305_Growing_foliose_lichens_on_cover_slips_A_method_for_asexual_propagation_and_observing_development
- Lichen cell factories: methods for the isolation of photobiont and ..., accessed May 21, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC9082883/
- Co-Cultivation of Fungal and Microalgal Cells as an Efficient System for Harvesting Microalgal Cells, Lipid Production and Wastewater Treatment | PLOS One, accessed May 21, 2025, https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0113497
Image References
- Kristian Peters -- Fabelfroh 09:10, 3 October 2006 (UTC), CC BY-SA 3.0, via Wikimedia Commons
- Ed Uebel, CC BY-SA 3.0, via Wikimedia Commons
- Ed Uebel, CC BY-SA 3.0, via Wikimedia Commons
Parmelia sp. - Waxpaper Lichen
| Parmelia Sp. of B.C. | |
|
Parmelia fraudans - Pea-green crottle [E-flora] |
Parmelia saxatilis - Pebbled crottle[E-flora] |
Parmelia fraudans
Status: Native [E-flora-LBC]-1
Habitat: Frequent over acid rock in cool, somewhat sheltered inland localities [E-flora-LBC]-1
World Distribution: incompletely circumpolar, S to AZ. [E-flora-LBC]-1
"General:
Medium stratified foliose lichens, corticate above and below, sorediate or isidiate or not, checkered pseudocyphellate (in BC),
lobes loosely attached to rather closely appressed, elongate, averaging to 1.5–10 mm wide, thin. Upper surface whitish or pale
greyish blue, somewhat shiny. Lower surface blackening, shiny, bearing short or rather long, simple or branched rhizines.
Medulla white. Photobiont green. Apothecia located over upper surface, disc brown; spores simple, ellipsoid, colourless,
8 per ascus. Over rock and trees." [E-flora-LBC]-1
"Notes: Parmelia is primarily a boreal and temperate genus consisting of 39 species worldwide. Eight of these are reported for North America and seven occur in B.C.
As originally circumscribed by Acharius in 1803, Parmelia encompassed an enormous assemblage of foliose lichens, including Lobaria, Pannaria and Xanthoria.
Beginning in the latter half of the 19th century, and apparently concluding only in the past decade, lichenologists have divided Parmelia into dozens of new
genera, most of which are now widely accepted. Local species accommodated until recently in Parmelia are now dispersed among the following genera: Ahtiana,
Arctoparmelia, Flavopunctelia, Hypotrachyna, Melanelia, Neofuscelia, Punctelia, and Xanthoparmelia." [E-flora-LBC]-1
"Species description:
Soredia and/or isidia present (rarely sparse); ecology various AND Thallus isidiate; isidia hard
and shiny or occasionally with soft, cottony appearance but never associated with soralia AND Rhizines
unbranched or at most sparsely forking/dichotomous; distribution various AND Isidia “soft”, not at
all hard-corticate, never shiny, generally short and granular; medulla K+ yellow becoming orange AND
Upper surface faintly yellowish; isidia mostly marginal, densely clustered" [E-flora-LBC]-1
Biochemicals:
"Atranorin, protolichesterinic acid, salazinic acid, usnic acid (soredia only)." [E-flora-LBC]-1
Image Reference
Jason Hollinger, CC BY-SA 3.0, via Wikimedia CommonsParmelia hygrophila
Status: Native [E-flora-LBC]-2
Habitat: "Common over trees in coastal and intermontane (ICH zone) forests, also rare over base-rich rock" [E-flora-LBC]-2
World Distribution: western N Am, N to AK, S to OR. [E-flora-LBC]-2
"General: Medium stratified foliose lichens, corticate above and below, sorediate or isidiate or not, checkered pseudocyphellate (in BC), lobes loosely attached to rather closely appressed, elongate, averaging to 1.5–10 mm wide, thin. Upper surface whitish or pale greyish blue, somewhat shiny. Lower surface blackening, shiny, bearing short or rather long, simple or branched rhizines. Medulla white. Photobiont green. Apothecia located over upper surface, disc brown; spores simple, ellipsoid, colourless, 8 per ascus. Over rock and trees." [E-flora-LBC]-2
"Notes: Parmelia is primarily a boreal and temperate genus consisting of 39 species worldwide. Eight of these are reported for North America and seven occur in B.C. As originally circumscribed by Acharius in 1803, Parmelia encompassed an enormous assemblage of foliose lichens, including Lobaria, Pannaria and Xanthoria. Beginning in the latter half of the 19th century, and apparently concluding only in the past decade, lichenologists have divided Parmelia into dozens of new genera, most of which are now widely accepted. Local species accommodated until recently in Parmelia are now dispersed among the following genera: Ahtiana, Arctoparmelia, Flavopunctelia, Hypotrachyna, Melanelia, Neofuscelia, Punctelia, and Xanthoparmelia." [E-flora-LBC]-2
"Species description:
Soredia and/or isidia present (rarely sparse); ecology various AND Thallus isidiate;
isidia hard and shiny or occasionally with soft, cottony appearance but never associated
with soralia AND Rhizines unbranched or at most sparsely forking/dichotomous; distribution
various AND Isidia “soft”, not at all hard-corticate, never shiny, generally short and
granular (but occasionally elongate in P. hygrophila); medulla K+ yellow becoming orange
AND Upper surface pale bluish grey to more often greenish; isidia mostly restricted to
upper surface, only rather weakly clustered; over bark (rare over rock);
widespread in humid regions" [E-flora-LBC]-2
Biochemicals:
"Atranorin and salazinic acid." [E-flora-LBC]-2
Image Reference
Jason Hollinger, CC BY 2.0, via Wikimedia CommonsParmelia omphalodes
Subtaxa Present in B.C.
- Parmelia omphalodes ssp. omphalodes [E-flora-LBC]-3
Habitat: Infrequent over acid rock in open sites throughout" [E-flora-LBC]-3
World Distribution: circumpolar, S to MT." [E-flora-LBC]-3
"General: Medium stratified foliose lichens, corticate above and below, sorediate or isidiate or not, checkered pseudocyphellate (in BC), lobes loosely attached to rather closely appressed, elongate, averaging to 1.5–10 mm wide, thin. Upper surface whitish or pale greyish blue, somewhat shiny. Lower surface blackening, shiny, bearing short or rather long, simple or branched rhizines. Medulla white. Photobiont green. Apothecia located over upper surface, disc brown; spores simple, ellipsoid, colourless, 8 per ascus. Over rock and trees." [E-flora-LBC]-3
"Notes: Parmelia is primarily a boreal and temperate genus consisting of 39 species worldwide. Eight of these are reported for North America and seven occur in B.C. As originally circumscribed by Acharius in 1803, Parmelia encompassed an enormous assemblage of foliose lichens, including Lobaria, Pannaria and Xanthoria. Beginning in the latter half of the 19th century, and apparently concluding only in the past decade, lichenologists have divided Parmelia into dozens of new genera, most of which are now widely accepted. Local species accommodated until recently in Parmelia are now dispersed among the following genera: Ahtiana, Arctoparmelia, Flavopunctelia, Hypotrachyna, Melanelia, Neofuscelia, Punctelia, and Xanthoparmelia." [E-flora-LBC]-3
"Species description: Soredia and isidia absent; over rock" [E-flora-LBC]-3
"Comments: The B.C. material can be assigned to ssp. omphalodes (Skult 1987)." [E-flora-LBC]-3
Biochemicals:
"Atranorin, protolichesterinic acid, salazinic acid." [E-flora-LBC]-3
Image Reference
Ed Uebel, CC BY-SA 3.0, via Wikimedia CommonsParmelia pseudosulcata
Status: Native [E-flora-LBC]-4
Habitat: Infrequent over conifers in lowland coastal forests, also rare in intermontane forests (ICH zone)" [E-flora-LBC]-4
World Distribution: western N Am, N to AK, S to CA." [E-flora-LBC]-4
"General: Medium stratified foliose lichens, corticate above and below, sorediate or isidiate or not, checkered pseudocyphellate (in BC), lobes loosely attached to rather closely appressed, elongate, averaging to 1.5–10 mm wide, thin. Upper surface whitish or pale greyish blue, somewhat shiny. Lower surface blackening, shiny, bearing short or rather long, simple or branched rhizines. Medulla white. Photobiont green. Apothecia located over upper surface, disc brown; spores simple, ellipsoid, colourless, 8 per ascus. Over rock and trees." [E-flora-LBC]-4
"Notes: Parmelia is primarily a boreal and temperate genus consisting of 39 species worldwide. Eight of these are reported for North America and seven occur in B.C. As originally circumscribed by Acharius in 1803, Parmelia encompassed an enormous assemblage of foliose lichens, including Lobaria, Pannaria and Xanthoria. Beginning in the latter half of the 19th century, and apparently concluding only in the past decade, lichenologists have divided Parmelia into dozens of new genera, most of which are now widely accepted. Local species accommodated until recently in Parmelia are now dispersed among the following genera: Ahtiana, Arctoparmelia, Flavopunctelia, Hypotrachyna, Melanelia, Neofuscelia, Punctelia, and Xanthoparmelia." [E-flora-LBC]-4
"Species description: Soredia and/or isidia present (rarely sparse); ecology various AND Thallus isidiate; isidia hard and shiny or occasionally with soft, cottony appearance but never associated with soralia AND Rhizines unbranched or at most sparsely forking/dichotomous; distribution various AND Isidia distinctly hard-corticate, often somewhat shiny, granular to long-cylindrical (in doubtful cases, check young isidia); medulla K- or K+ yellow becoming orange AND Lobes more or less closely appressed, seldom overlapping; rhizines rather freely forking/dichotomous; over bark; medulla K-" [E-flora-LBC]-4
"Reactions: Cortex K+ yellow, medulla PD+ orange to red." [E-flora-LBC]-4
Biochemicals: "Atranorin, lobaric acid and protocetraric acid." [E-flora-LBC]-4
Syn
Parmelia kerguelensis auct. [E-flora]
Parmelia saxatilis
 |
 |
| Parmelia saxatilis | Parmelia saxatilis |
Status: Native [E-flora]-5
Habitat: Common over acid rock in open sites throughout, also infrequent over conifers in open coastal forests; widespread" [E-flora-LBC]-5
World Distribution: circumpolar. [E-flora-LBC]-5"General: Medium stratified foliose lichens, corticate above and below, sorediate or isidiate or not, checkered pseudocyphellate (in BC), lobes loosely attached to rather closely appressed, elongate, averaging to 1.5–10 mm wide, thin. Upper surface whitish or pale greyish blue, somewhat shiny. Lower surface blackening, shiny, bearing short or rather long, simple or branched rhizines. Medulla white. Photobiont green. Apothecia located over upper surface, disc brown; spores simple, ellipsoid, colourless, 8 per ascus. Over rock and trees." [E-flora-LBC]-5
"Notes: Parmelia is primarily a boreal and temperate genus consisting of 39 species worldwide. Eight of these are reported for North America and seven occur in B.C. As originally circumscribed by Acharius in 1803, Parmelia encompassed an enormous assemblage of foliose lichens, including Lobaria, Pannaria and Xanthoria. Beginning in the latter half of the 19th century, and apparently concluding only in the past decade, lichenologists have divided Parmelia into dozens of new genera, most of which are now widely accepted. Local species accommodated until recently in Parmelia are now dispersed among the following genera: Ahtiana, Arctoparmelia, Flavopunctelia, Hypotrachyna, Melanelia, Neofuscelia, Punctelia, and Xanthoparmelia." [E-flora-LBC]-5
"Species description: Soredia and/or isidia present (rarely sparse); ecology various AND Thallus isidiate; isidia hard and shiny or occasionally with soft, cottony appearance but never associated with soralia AND Rhizines unbranched or at most sparsely forking/dichotomous; distribution various AND Isidia distinctly hard-corticate, often somewhat shiny, granular to long-cylindrical (in doubtful cases, check young isidia); medulla K- or K+ yellow becoming orange AND Lobes often rather loosely attached and overlapping; rhizines mostly unbranched; over rock (rare over bark); medulla K+ yellow becoming orange" [E-flora-LBC]-5
"Comments: Included under Parmelia saxatilis are two rather distinct morphologies that may deserve separate taxonomic recognition. These are distinguished as follows: 1. Parmelia saxatilis - Upper surface pale bluish grey (brownish where exposed), generally pale or concave; pseudocyphellae in netlike patterns throughout; isidia generally originating in part over upper surface. 2. Parmelia sp. 1 - Upper surface greenish (but turning yellowish in herbarium), often convex; pseudocyphellae generally confined to area of lobe tips; isidia tending at first to arise along lobe margins (though later also developing over upper surface)" [E-flora-LBC]-5
"Reactions: Cortex K+ yellow, medulla K+ yellow becoming red, PD+ orange to red." [E-flora-LBC]-5
Biochemicals: "Atranorin, lobaric acid and salazinic acid." [E-flora-LBC]-5
Syn
Parmelia kerguelensis A. Wilson [E-flora-5]
Image Reference
Jerzy Opioła, CC BY-SA 3.0, via Wikimedia Commonsjensu, CC0, via Wikimedia Commons
Parmelia skultii - Silver-rimmed crottle
Status: Native [E-flora]-6
Parmelia squarrosa - bottlebrush crottle
Red-Listed in B.C.
Status: Native [E-flora]-7
"Habitat: Infrequent over conifers in open coastal forests at lower elevations" [E-flora-LBC]-7
"World Distribution: incompletely circumpolar, S to CA." [E-flora-LBC]-7
"General: Medium stratified foliose lichens, corticate above and below, sorediate or isidiate or not, checkered pseudocyphellate (in BC), lobes loosely attached to rather closely appressed, elongate, averaging to 1.5–10 mm wide, thin. Upper surface whitish or pale greyish blue, somewhat shiny. Lower surface blackening, shiny, bearing short or rather long, simple or branched rhizines. Medulla white. Photobiont green. Apothecia located over upper surface, disc brown; spores simple, ellipsoid, colourless, 8 per ascus. Over rock and trees." [E-flora-LBC]-7
"Notes: Parmelia is primarily a boreal and temperate genus consisting of 39 species worldwide. Eight of these are reported for North America and seven occur in B.C. As originally circumscribed by Acharius in 1803, Parmelia encompassed an enormous assemblage of foliose lichens, including Lobaria, Pannaria and Xanthoria. Beginning in the latter half of the 19th century, and apparently concluding only in the past decade, lichenologists have divided Parmelia into dozens of new genera, most of which are now widely accepted. Local species accommodated until recently in Parmelia are now dispersed among the following genera: Ahtiana, Arctoparmelia, Flavopunctelia, Hypotrachyna, Melanelia, Neofuscelia, Punctelia, and Xanthoparmelia." [E-flora-LBC]-7
"Species description: Soredia and/or isidia present (rarely sparse); ecology various AND Thallus isidiate; isidia hard and shiny or occasionally with soft, cottony appearance but never associated with soralia AND Rhizines side-branched/squarrose when mature; restricted to coastal localities" [E-flora-LBC]-7
"Reactions: Cortex K+ yellow, medulla K+ yellow becoming red, PD+ orange." [E-flora-LBC]-7
Biochemicals: "Atranorin and salazinic acid." [E-flora-LBC]-7
Parmelia sulcata - Hammered crottle
Status: Native [E-flora]-8
"Habitat / Range Habitat: Common over trees throughout, also infrequent over acid rock" [E-flora-LBC]-8
"World Distribution: circumpolar, N to AK, S to CA." [E-flora-LBC]-8
"General: Medium stratified foliose lichens, corticate above and below, sorediate or isidiate or not, checkered pseudocyphellate (in BC), lobes loosely attached to rather closely appressed, elongate, averaging to 1.5–10 mm wide, thin. Upper surface whitish or pale greyish blue, somewhat shiny. Lower surface blackening, shiny, bearing short or rather long, simple or branched rhizines. Medulla white. Photobiont green. Apothecia located over upper surface, disc brown; spores simple, ellipsoid, colourless, 8 per ascus. Over rock and trees." [E-flora-LBC]-8
"Notes:: Parmelia is primarily a boreal and temperate genus consisting of 39 species worldwide. Eight of these are reported for North America and seven occur in B.C. As originally circumscribed by Acharius in 1803, Parmelia encompassed an enormous assemblage of foliose lichens, including Lobaria, Pannaria and Xanthoria. Beginning in the latter half of the 19th century, and apparently concluding only in the past decade, lichenologists have divided Parmelia into dozens of new genera, most of which are now widely accepted. Local species accommodated until recently in Parmelia are now dispersed among the following genera: Ahtiana, Arctoparmelia, Flavopunctelia, Hypotrachyna, Melanelia, Neofuscelia, Punctelia, and Xanthoparmelia." [E-flora-LBC]-8
"Species description: Soredia and/or isidia present (rarely sparse); ecology various AND Thallus sorediate; soredia dull, confined to discrete soralia" [E-flora-LBC]-8
"Reactions: Cortex K+ yellow, medulla K+ yellow becoming red, PD+ orange." [E-flora-LBC]-8
Biochemistry: "Atranorin and salazinic acid." [E-flora-LBC]-8
"Habitat: "Perhaps the most eurysubstratic lichen is Parmelia sulcata, found almost everywhere in temperate and boreal regions of the world. It is apparently equally tolerant of bark, stone, and wood, and is known not infrequently from soil as well. Perhaps more important, it apparently has no strong preference with regard to neutral versus acidic substrates, although I have never seen it on limestone." [Ahmadjidan Lichens]
Net-marked parmelia (Parmelia sulcata), or Shield lichen, is a foliose lichen in the family Parmeliaceae. It is very tolerant of pollution and has a cosmopolitan distribution, making it one of the most common lichens. It harbours a unicellular Trebouxia green algal symbiont.[????]
Medicinal Uses
It is significant that another species of the same genus--Parmelia sulcata, which is described in medicinal plant lore of India--is also reported as medicinally useful by Hale in cranial maladies. Lipp highlighted (13) the work of Curtin (14) who wrote that Piman informants likened the effect of a lichen to that of marijuana and noted that the smoking of this lichen "makes young men crazy." [ParmeliaLUse]
- Metis "Rubbed on gums of teething babies to relieve discomfort (Marles et al. 2000)" [Rankovic]
- Saanich "Medicinal properties depend on type of tree it is growing on. Possible the lichen traditionally used for birth control. Not differentiated from Lobaria pulmonaria (Turner and Hebda 2012)" [Rankovic]
- "...Parmelia sulcata Taylor (Parmeliaceae) have been used in the treatment of pulmonary and cranial diseases, respectively (Rizzini 1952)." [Rai MPBD]
Lore Species such as Lobaria pulmonaria or Parmelia sulcata were widely used in the Middle Ages owing to the "signature theory" prevalent in those times. This theory related the morphological and ecological characteristics of the plants with the ailment which was to be cured. For this reason the scrobiculate thallus of Lobaria pulmonaria was used to treat pulmonary illnesses and Parmelia cranial diseases.[3LUPM]
Activities
- Antibaterial & Selectively Antifungal: "The acetone and methanol extracts of ... Parmelia sulcata ... manifested antibacterial activity against the majority of species of bacteria tested, in addition to selective antifungal activity. Acetone, chloroform, diethyl ether, methanol, and petroleum ether extracts of Parmelia sulcata and its constituent (salazinic acid) demonstrated antibacterial activity against Aeromonas hydrophila, Bacillus cereus, Bacillus subtilis, Listeria monocytogenes, Proteus vulgaris, Yersinia enterocolitica, Staphylococcus aureus, Streptococcus faecalis, Candida albicans, Candida glabrata, Aspergillus niger, Aspergillus fumigatus, and Penicillium notatum." [????]
Misc
" In contrast, the epiphytic lichen Parmelia sulcata (also a common genus in soil crusts) acclimated after only 30 days to 700 ppm CO2 (Balaguer et al., 1996). When subsequently exposed to 350 ppm CO2, photosynthetic capacity was reduced, associated with less Rubisco present in the pyrenoid of the algal chloroplasts. The efficiency of photosystem II photochemistry was not significantly changed." [Dighton TFC]
Bioaccumulation
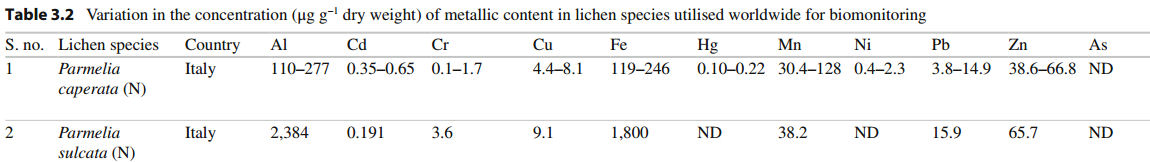
[Shukla LBE]
"Brown et al. [21] showed that particulates trapped between the lichen thallus and bark may play a role in supplying elements to lichens, e.g. Parmelia sulcata growing directly on bark contained higher concentrations of AI, Cr, Fe and Mg than did samples growing over epiphytic mosses.Moreover, levelsofthe same elements were much lower in adjacent surface bark uncolonised by lichens than in debris entrapped between the analysed thallus and the underlying bark. These results indicated that the presence of the lichen leads to the accumulation of particulate elements beneath the thallus (i.e. lichens act as a filter for particles washed down the tree-trunkby stem flow)" [Nimis ML]
- http://linnet.geog.ubc.ca/Atlas/Atlas.aspx?sciname=Parmelia%20sulcata&redblue=Both&lifeform=11, Accessed March 14, 2015
- [2] Personal Observation and notes.
- [ParmeliaLUse] Parmelia spp. (lichens) in ancient medicinal plant lore of India, Kumar, Kaushal, D. K. Upreti, India, ECONOMIC BOTANY Vol 55
- http://en.wikipedia.org/wiki/Parmelia_sulcata, Accessed March 14, 2015
Uses of Parmelia Sp.
Lore
Our literary review and records of medicinal
plant lore of India show the word Shipal is used
for algae in Rigveda (6000-4000 B.C.) (5), a text where the first authentic record of Oshadhi
(medicine) has been described. The medicinal
properties attributed to Shipal as a lichen are in
Avkolva as mentioned in Athraveda (1500 B.C.)
(6). Subsequently a number of Sanskrit synonyms of lichens--for example, Shailaya, and
Shilapushp--have been described in Sushruta
Samhita (1000 B.C.), Charak Samhita (300-200
B.C.), and several Nighantu (A.D. 1100-1800) (7,
8). The work in the above classical texts is followed by Sharma who carried out their critical
analysis (9, 10). The Sanskrit names of lichens
were later identified to several species of the genus Parmelia (11, 12) such as P. cirrhata and
P. perforata. The vernacular name Chharlia is
widely used in Ayurveda, an ancient system of
medicine in India, for different diseases and disorders, for example, headache, skin diseases,
urinary trouble, boils, vomiting, diarrhoea, dysentery, heart trouble, cough, fever, leprosy, and
as a blood purifier.[ParmeliaLUse]
Other Usage
Litmus Litmus can be found in different species of lichens. The dyes are extracted from such species as ...Parmelia. Currently, the main sources are Roccella montagnei (Mozambique) and Dendrographa leucophoea (California).[1]
The main use of litmus is to test whether a solution is acidic or basic. Wet litmus paper can also be used to test for water-soluble gases that affect acidity or alkalinity; the gas dissolves in the water and the resulting solution colors the litmus paper. For instance, ammonia gas, which is alkaline, colors the red litmus paper blue.
Blue litmus paper turns red under acidic conditions and red litmus paper turns blue under basic or alkaline conditions, with the color change occurring over the pH range 4.5-8.3 at 25 °C (77 °F). Neutral litmus paper is purple.[1] Litmus can also be prepared as an aqueous solution that functions similarly. Under acidic conditions, the solution is red, and under basic conditions, the solution is blue. [Wiki]
Medicinal Usage
PARMELIA (Alpine Lichen)
LICHEN.
Tincture [1:5, 50% alcohol] 30-60 drops to 4X a day. Moisten the
herb with a little alcohol and make a Strong Decoction, 2-6 ounces, to 3X a
day, or use the tea for topical application.
STATUS : W/LA [Moore (1995)]
“Parmelia sulcata Taylor (Parmeliaceae) have been used in the treatment of pulmonary and cranial diseases, respectively."
(Rizzini, 1952[Rizzini])”
[ISPUB]S. Malhotra, R. Subban, A. Singh: Lichens- Role in Traditional Medicine and Drug Discovery. The Internet Journal of Alternative Medicine. 2008 Volume 5 Number 2. DOI: 10.5580/3d9
According to one Irish source,33 a very common lichen in Sligo variously known there as ‘tree lungwort’,‘hazel rag’ or ‘crottles’ has been used locally as a cure for piles. That sounds like a conflation of more than one kind and perhaps refers more particularly to Parmelia species, which have been put to related purposes. [MPFT]
Three Parmelia sp. (Parmelia chinense (Osbeck) Hale & Ahti., syn P. perlata (Huds.) Ach. P. sancti-angeli (Lynge) Hale and P. peforatum (Jacq.) A. Massal. are used for the Indian drug chharila, which is used as an aphrodisiac. In India Parmelia chinense is used as a diuretic and as a liniment for headaches and powder to help wounds heal. Parmelia sanctiangeli is used in Central India to treat Tinea (ringworm) disease. Ash of the lichen, mixed with mustard or linseed oil, is applied to the affected area. Parmelia peforatum is medically recognized in Afghanistan. Parmelia nepalense (Talyor) Hale ex Sipman is used in Nepal in the treatment of toothache and sore throat.[????]
Hazards:
More recently a report of the Wyoming
Agricultural Experiment Station on a
study of the presence of selenium in soil
and various plants states that Parmelia
molliuscula Ach. contains this poisonous
salt in sufficient quantities to affect sheep
and cattle. It produces a lack of coordination of the hind limbs; in severe cases
the animals are unable to move either
hind or fore legs. Other examples of
lichens containing such elements include
beryllium in Parmelia saxatilis Ach.[EUL]
Related Species
- Parmelia physodes (L.) Ach., Uchen · Food-Potawatomi soup and Vegetable Cooked into a soup, materi- al swelled and afforded a pleasant flavor. (154:107) Parmelia saxatilis (L.) Ack., Flat Uchen · Fiber-Eskimo, Inuktitut Canoe Material Used to stuff caribou skins for rafts. (202:190) [NAEth Moerman]
- In
India (17) Parmelia abessinica, "Rathipuvvu", is used as food, generally in a
curry powder, and medicinally.[EUL]
Parmelia molliuscula Ach. Ground Lichen. 1. Ni'xat16,at (earth cover- ing). 2. Tshétldat (rock covering). This stone lichen may be scraped from the rocks after a rain and made into a yellow-orange dye (26:182). It is also used as a remedy for impetigo.[Elmore EON]
- The use of lichens as remedies for sore mouth or gums is a widespread practice.26 Plants used for canker,
swollen gums, decayed teeth, etc., are chewed.
Parmelia molliuscula (2), Peltigera sp. (5), Mirabilis spp. (2), Aster ericafolius (3), Sanvitalia Aberti.[Wyman NIME]
- Puffed shield lichen - Parmelia physodes - Boiled in soups by the Potawatomi.
Lichen (Parmelia physodes [L.] Ach.) which grows upon a spruce tree, shown in plate 21, fig. 2, “wakwûnûk” [egg bush]. The Potawatomi only use lichens that are found upon spruce trees and while they are apt to eat it as they find it in the woods, as a cure for constipation, it was usually soaked or boiled in water until it swelled somewhat. It is also used as a food.[HuronSmith Zuni]
- Lichen (Parmelia physodes [L.] Ach.) shown in plate 21, fig. 2,
“wa'kwûnûk” [egg bush]. The Lichen that grows upon a spruce tree, as
our specimen did, is gathered by the Forest Potawatomi for a vegetable
soup material. When it is cooked into a soup it swells somewhat and
affords a pleasant flavor.[HuronSmith Zuni]
- Up to 1,000 tons/year of Parmelia nepalensis (Taylor) Hale ex Sipman is
processed into lichen oil, absolute or extract in Western Nepal, and exported for
global perfumery and incense use (although the lichens are also used in
traditional systems of medicine) – Cropwatch Newsletter Aug 2008.[OakmossBiblio]
- During ethnobotanical research among the Papago, samples of this lichen were obtained and later identified as P. conspera. Considerable magical potency is attributed to this plant by the Papago-Pima, since it is also used for success in hunting, in gambling, in love, and for disposing of an enemy. He also quoted the reference of Devereux, Vestal, and Schultes (15, 16) and stated that whether these specimens account for the use of lichens with similar properties by the Mohave and Kiowa or whether other lichen genera are involved can not be presently ascertained.[ParmeliaLUse]
Phytochemicals
A phenolic derivative, atraric
2
acid, was identified by GC/MS and quantified by GC/MS/MS in the outer parts of
wood from oaks specifically colonised by lichens. This compound was correlated
to the presence of a bitter depside, atranorin, which is a natural metabolite of the
lichen species belonging to the genus Parmelia.[OakmossBiblio]
Treemoss
(Mousse d’arbre) Treemoss derivatives (concretes, absolutes) are mainly
prepared from the lichen species Pseudevernia furfuracea (L.) Zopf. with Usnea
barbata, Parmelia sulcata and other species often co-gathered in. These tree
lichens can both be found living on the barks of firs and pines in Southern and
Central Europe including and France and Morocco, & Balkan countries, including
former Yugoslavia. Preparation of fragrant treemoss products is carried out in a
similar manner to the preparation of oakmoss products, although evidence that
isopropanol may be included as a processing solvent is shown by the presence
of isopropyl haematommate (which does not exist in lichens) in the analysis of
the weakly acidic factions of treemoss absolute (Endo et al. 1999). It should be
noted that Treemoss products are generally considered inferior to oakmoss
products and command a lower purchasing price – Cropwatch Newsletter Aug.
2008.[OakmossBiblio]
A number of bromine-containing polyacetylenic methyl esters are found in some lichen species e.g., (36) from the Parmelia species [CRC TLHB]
References
- (E-flora) In Klinkenberg, Brian. (Editor) 2020. E-Flora BC: Electronic Atlas of the Plants of British Columbia
[eflora.bc.ca]. Lab for Advanced Spatial Analysis, Department of Geography, University of British Columbia, Vancouver.
[Accessed: 2025-05-28]
- LBC - Lichens of British Columbia
- 1 - Parmelia fraudans, https://linnet.geog.ubc.ca/Atlas/Atlas.aspx?sciname=Parmelia%20fraudans&redblue=Both&lifeform=11
- 2 - Parmelia hygrophila, https://linnet.geog.ubc.ca/Atlas/Atlas.aspx?sciname=Parmelia%20hygrophila&redblue=Both&lifeform=11
- 3 - Parmelia omphalodes, https://linnet.geog.ubc.ca/Atlas/Atlas.aspx?sciname=Parmelia%20omphalodes&redblue=Both&lifeform=11
- 4 - Parmelia pseudosulcata, https://linnet.geog.ubc.ca/Atlas/Atlas.aspx?sciname=Parmelia%20pseudosulcata&redblue=Both&lifeform=11
- 5 - Parmelia saxatilis, https://linnet.geog.ubc.ca/Atlas/Atlas.aspx?sciname=Parmelia%20saxatilis&redblue=Both&lifeform=11
- 6 - Parmelia skultii, https://linnet.geog.ubc.ca/Atlas/Atlas.aspx?sciname=Parmelia%20skultii&redblue=Both&lifeform=11
- 7 - Parmelia squarrosa, https://linnet.geog.ubc.ca/Atlas/Atlas.aspx?sciname=Parmelia%20squarrosa&redblue=Both&lifeform=11
- 8 - Parmelia sulcata, https://linnet.geog.ubc.ca/Atlas/Atlas.aspx?sciname=Parmelia%20sulcata&redblue=Both&lifeform=11
- (EUL)Economic Uses of Lichens, GEORGE A. LLANO
- [Wiki] http://en.wikipedia.org/wiki/Litmus Accessed March 14, 2015


